Michael E. Wechsler, MD, MMSc
Professor of Medicine, Division of Pulmonary, Critical Care, and Sleep Medicine
Director, National Jewish Health/Cohen Family Asthma Institute
National Jewish Health
Denver, Colorado
Dr. Michael Wechsler is Professor of Medicine in the Division of Pulmonary, Critical Care, and Sleep Medicine at National Jewish Health (NJH) in Denver, Colorado, and Director of the NJH/Cohen Family Asthma Institute. In addition to clinical work in pulmonary and critical care medicine, Dr. Wechsler’s research focuses on clinical and translational asthma with an emphasis on clinical trials in asthma, novel asthma therapies, bronchial thermoplasty, asthma pharmacogenomics, and management of eosinophilic granulomatosis with polyangiitis (ie, Churg-Strauss Syndrome [CSS]). He has led studies focusing on novel biologic agents for asthma and related diseases, including benralizumab, dupilumab, mepolizumab, reslizumab, tezepelumab, and depemokimab. He has published more than 340 manuscripts relating to asthma, eosinophilic granulomatosis with polyangiitis (EGPA), and eosinophilic lung diseases; he has also been an investigator in over 60 clinical trials. He was a member of the Steering Committee and site Principal Investigator of the NIH-sponsored Asthma Clinical Research Network (ACRN/AsthmaNet), a multicenter asthma clinical trials consortium, and currently serves as the Principal Investigator of the Denver site of the Precision Intervention in Severe/Exacerbating Asthma (PRECISE) network. A member of the American Society of Clinical Investigation and the Association of American Physicians, he has participated in many different task forces related to the study of eosinophilic lung diseases that were sponsored by the NIH, the FDA, the European Respiratory Society, and the International Eosinophil Society. He is currently Associate Editor of the CHEST Journal and has served as Associate Editor of the journal, Allergy, and on the editorial board of the European Journal of Clinical Investigation Dr. Wechsler received BA and MMSc degrees from Harvard University in Boston, Massachusetts, and an MD degree from McGill University in Montreal. He completed medical training at Beth Israel Hospital in Boston and as part of the Harvard Combined Pulmonary and Critical Care Fellowship Training Program.

TARGET AUDIENCE
This activity is intended for rheumatologists and other specialists (eg, pulmonologists, allergists/clinical immunologists) who manage patients with eosinophilic granulomatosis with polyangiitis (EGPA).
PROGRAM OVERVIEW
Eosinophilic granulomatosis with polyangiitis (EGPA), formerly known as Churg-Strauss syndrome, is a rare autoimmune small-vessel vasculitis that has a complex phenotype with symptomatology that affects multiple organ systems. Join us for this engaging Expert Insights program to learn more about EGPA, an intriguing heterogeneous multisystem disease plagued by delays in diagnosis – and the effective targeted biologic therapies that are now available to treat this disease. Along with a whiteboard animation, multidisciplinary faculty will review the pathophysiologic processes underlying EGPA development, best approaches for prompt diagnosis, and strategies for individualizing therapeutic regimens that improve patient outcomes.
EDUCATIONAL OBJECTIVES
• Describe the pathophysiologic causes and biopsychosocial consequences of EGPA
• Identify patients with EGPA quickly based on symptoms, laboratory testing, and ruling out of other potential disorders
• Discuss the clinical rationale and trial evidence for biologic treatment options for patients with EGPA
• Construct biologic-based regimens for patients with EGPA to address underlying pathophysiologic processes, reduce corticosteroid exposure, and target remission
PROGRAM AGENDA
5:30 pm Registration/Dinner
6:00 pm Preactivity Questionnaire and Faculty Introductions
6:10 pm Burdens and Pathophysiology of EGPA
6:25 pm Diagnostic Challenges in EGPA
6:40 pm Evolving Management Recommendations for EGPA
6:55 pm Comprehensive EGPA Care
7:15 pm Postactivity Questionnaire and Q&A Session
Physician Accreditation Statement
Integritas Communications is accredited by the Accreditation Council for Continuing Medical Education (ACCME) to provide continuing medical education for physicians.
Physician Credit Designation
Integritas designates this live activity for a maximum of 1.5 AMA PRA Category 1 Credits™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Integritas Contact Information
For more information about the approval of this program, please contact Integritas at info@exchangecme.com.
Instructions to Receive Credit
In order to receive credit for this activity, the participant must attend the live symposium/live stream and complete the posttest and program evaluation.
Fee Information & Refund/ Cancellation Policy
There is no fee for this educational activity.
Disclosures of Conflicts of Interest
Integritas adheres to the policies and guidelines, including the Standards for Integrity and Independence in Accredited CE, set forth to providers by the Accreditation Council for Continuing Medical Education (ACCME) and all other professional organizations, as applicable, stating those activities where continuing education credits are awarded must be balanced, independent, objective, and scientifically rigorous. All persons in a position to control the content of an accredited continuing education program provided by Integritas are required to disclose all financial relationships with any ineligible company within the past 24 months to Integritas. All financial relationships reported are identified as relevant and mitigated by Integritas in accordance with the Standards for Integrity and Independence in Accredited CE in advance of delivery of the activity to learners. The content of this activity was vetted by Integritas to assure objectivity and that the activity is free of commercial bias.

All relevant financial relationships have been mitigated. The faculty have the following relevant financial relationships with ineligible companies:
Brian D. Jaros, MD: Consulting Fees: Novartis Pharmaceuticals Corporation; Non-CE Services: AbbVie Inc.; Contracted Research: Amgen Inc.
Philip Seo, MD, MHS: Non-CE Services: Amgen Inc.
Michael E. Wechsler, MD, MMSc: Consulting Fees: Allakos, Amgen, Areteia Therapeutics, Arrowhead Pharmaceutical, AstraZeneca, Avalo Therapeutics, Belenos Bio, Celldex, Connect Biopharma, Eli Lilly, Equillium, Glaxosmithkline, Incyte, Jasper Therapeutics, Kinaset, Kymera, Merck, MyBiometry, Pharming, Phylaxis, Pulmatrix, Rapt Therapeutics, recludix Pharma, Roche/ Genentech, Regeneron, Sanofi/Genzyme, Sentien, Sound Biologics, Tetherex Pharmaceuticals, Uniquity Bio, Upstream Bio, Verona Pharma, Zurabio; Stock Options: Upstream Bio
The planners and managers have no relevant financial relationships with ineligible companies.
Disclosure of Unlabeled Use
This educational activity may contain discussion of published and/ or investigational uses of agents that are not indicated by the US Food and Drug Administration. Integritas does not recommend the use of any agent outside of the labeled indications. The opinions expressed in the educational activity are those of the faculty and do not necessarily represent the views of any organization associated with this activity. Please refer to the official prescribing information for each product for discussion of approved indications, contraindications, and warnings.
Disclaimer
Participants have an implied responsibility to use the newly acquired information to enhance patient outcomes and their own professional development. The information presented in this activity is not meant to serve as a guideline for patient management. Any procedures, medications, or other courses of diagnosis or treatment discussed in this activity should not be used by clinicians without evaluation of patient conditions and possible contraindications or dangers in use, review of any applicable manufacturer’s product information, and comparison with recommendations of other authorities.
Americans With Disabilities Act
Event staff will be glad to assist you with any special needs (ie, physical, dietary, etc). Please contact us prior to the live event at info@exchangecme.com.
CLINICAL PRACTICE GUIDELINES
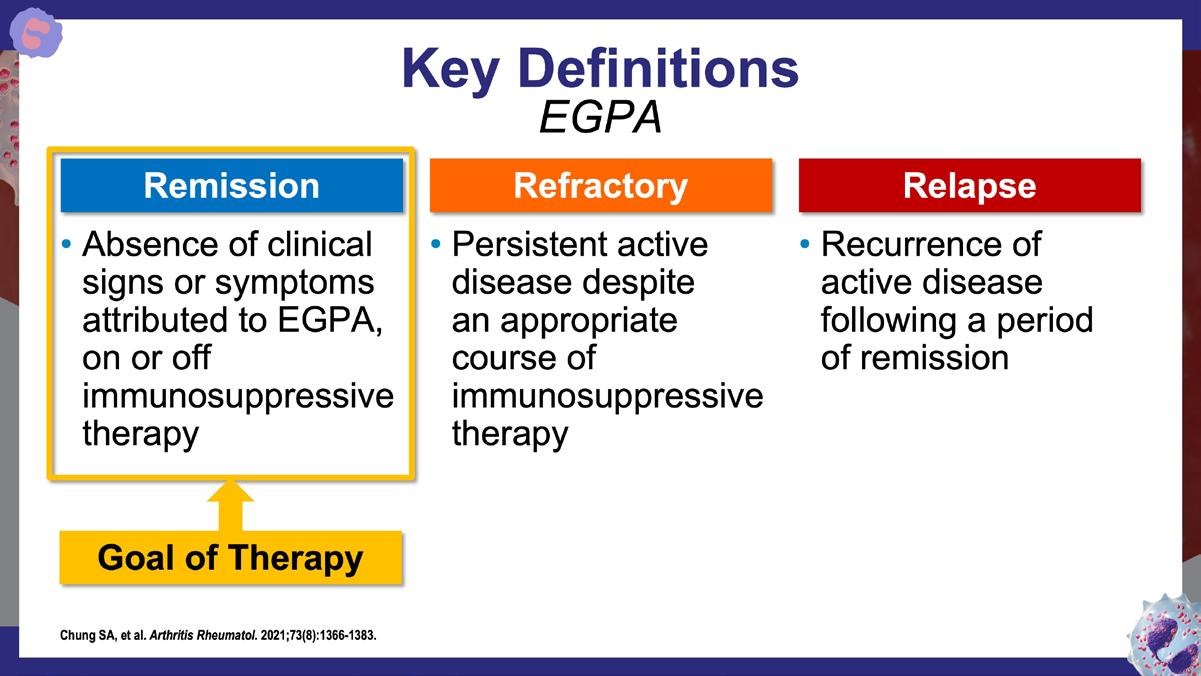
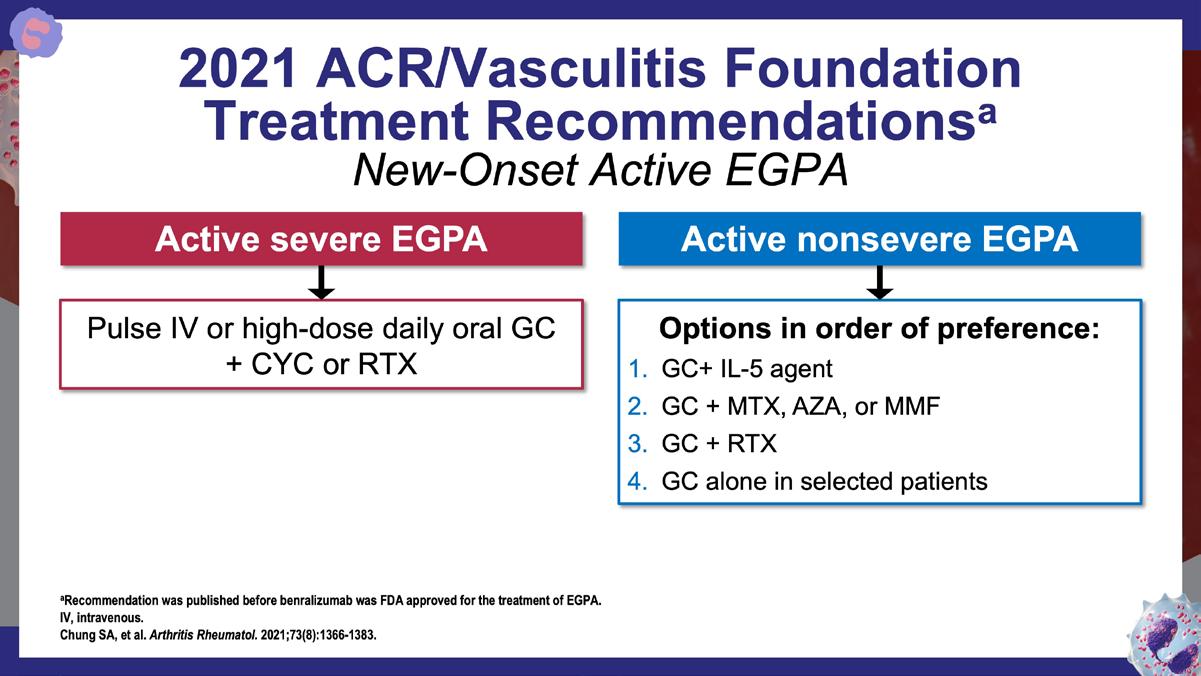
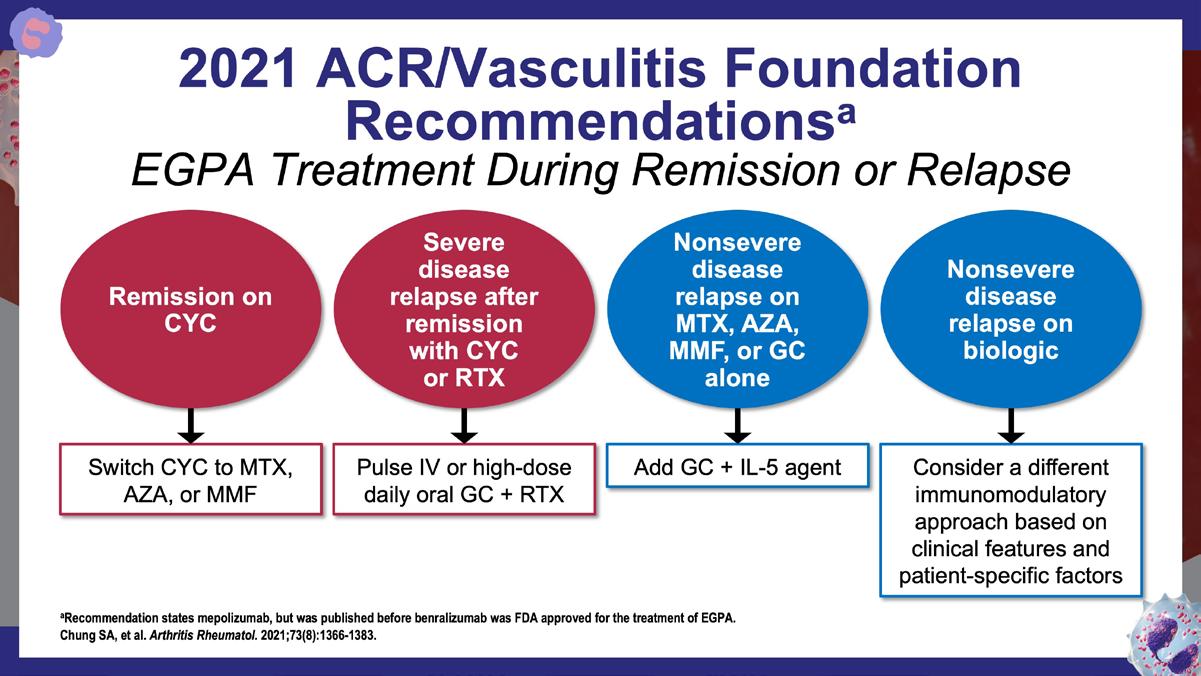
» 2021 American College of Rheumatology (ACR)/Vasculitis Foundation guideline for the management of antineutrophil cytoplasmic antibody–associated vasculitis.
Chung SA, et al. Arthritis Rheumatol. 2021;73(8):1366-1383. https://acrjournals.onlinelibrary.wiley.com/doi/10.1002/art.41773
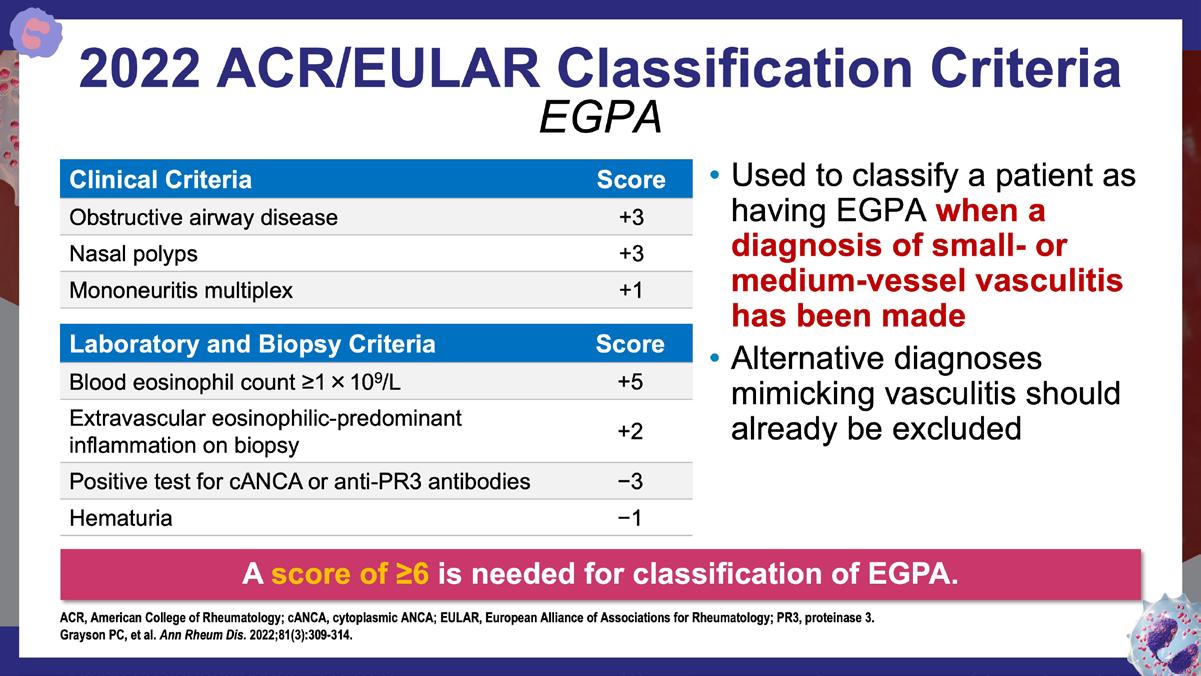
» 2022 American College of Rheumatology/ European Alliance of Associations for Rheumatology (EULAR) classification criteria for eosinophilic granulomatosis with polyangiitis.
Grayson PC, et al. Ann Rheum Dis. 2022;81(3):309-314. https://ard.eular.org/article/S0003-4967(24)09565-7/abstract
» EULAR recommendations for the management of ANCA- associated vasculitis: 2022 update.
Hellmich B, et al. Ann Rheum Dis. 2024;83(1):30-47. https://ard.bmj.com/content/83/1/30
SUGGESTED READING
» Evidence-based guideline for the diagnosis and management of eosinophilic granulomatosis with polyangiitis.
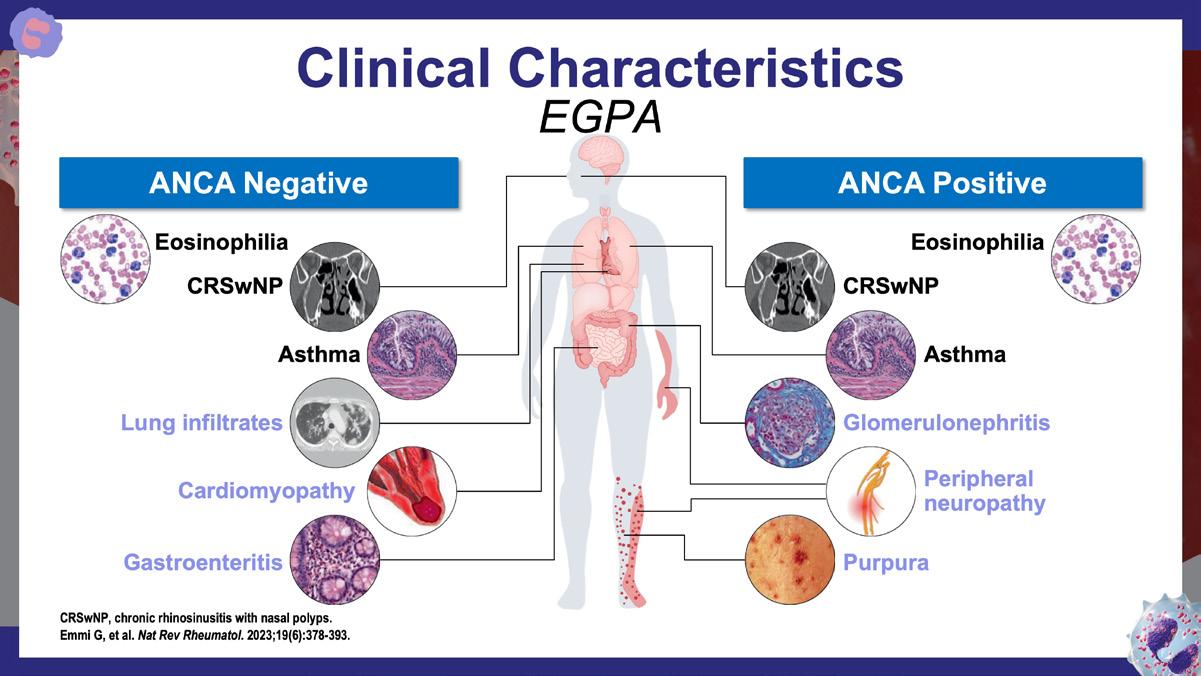
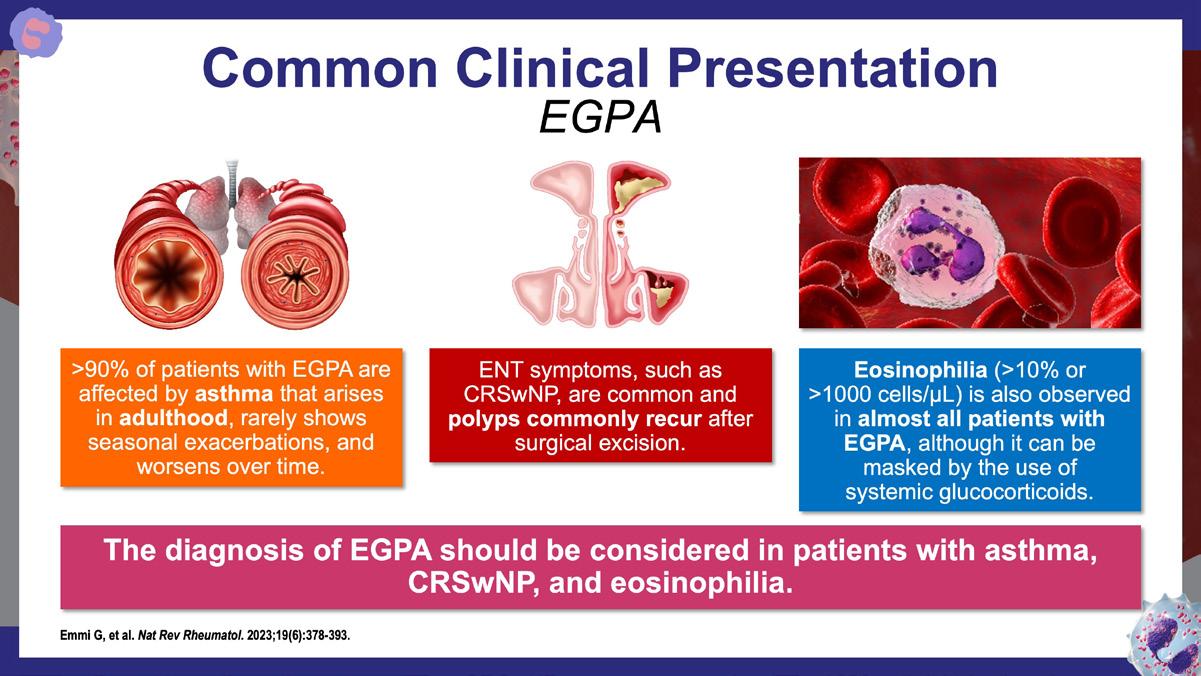
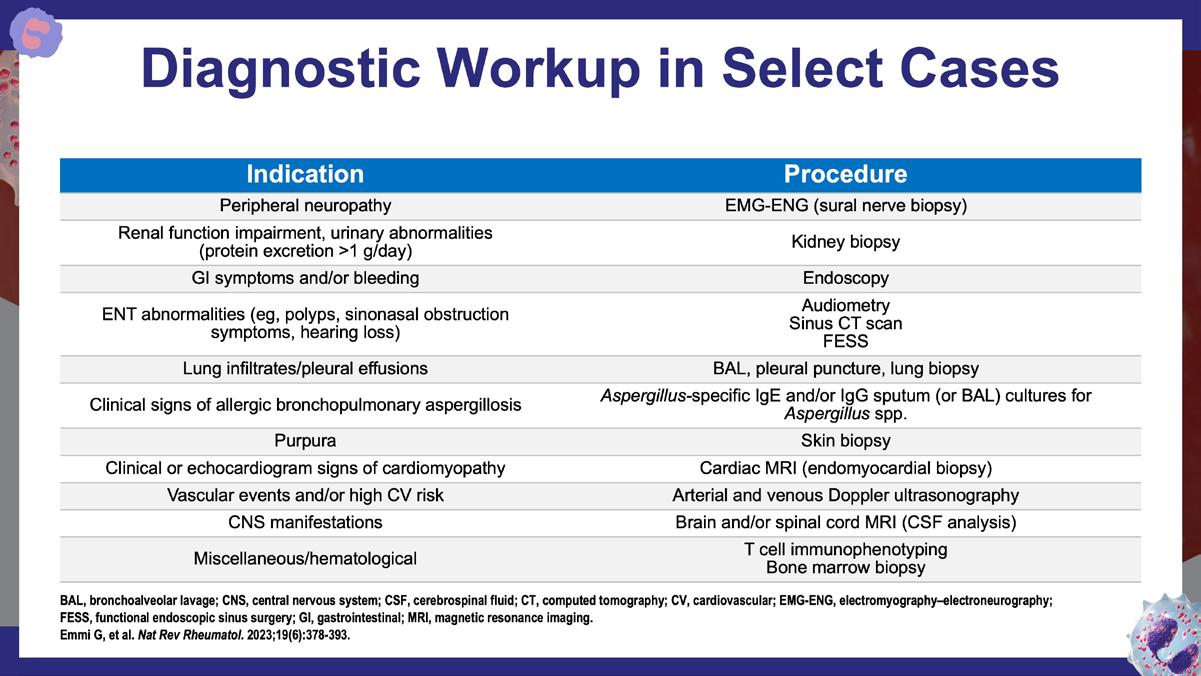
Emmi G., et al. Nat Rev Rheumatol. 2023;19(6):378-393. https://www.nature.com/articles/s41584-023-00958-w
» The five-factor score revisited: assessment of prognoses of systemic necrotizing vasculitides based on the French Vasculitis Study Group (FVSG) cohort.
Guillevin L, et al. Medicine. 2011;90(1):19-27. https://journals.lww.com/md-journal/fulltext/2011/01000/the_five_ factor_score_revisited__assessment_of.2.aspx
» Benralizumab as a steroid-sparing treatment option in eosinophilic granulomatosis with polyangiitis.
Gunter VP, et al. J Allergy Clin Immunol Pract. 2021;9(3):1186-1193. https://www.jaci-inpractice.org/article/S2213-2198(20)31104-1/abstract
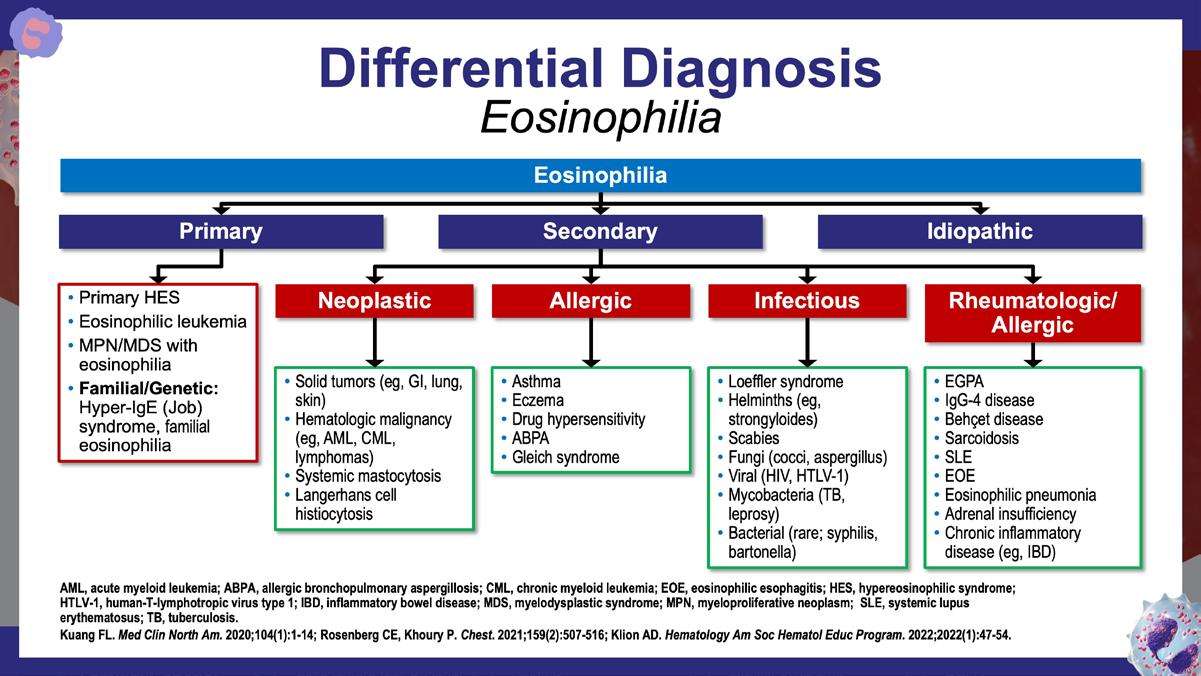
» Approach to the patient with suspected hypereosinophilic syndrome.
Klion AD. Hematology Am Soc Hematol Educ Program. 2022;2022(1):47-54.
https://pmc.ncbi.nlm.nih.gov/articles/PMC9821533/
» Red flags for clinical suspicion of eosinophilic granulomatosis with polyangiitis (EGPA).
Solans-Laqué R, et al. Eur J Intern Med. 2024;128:45-52.
https://www.ejinme.com/article/S0953-6205(24)00247-4/fulltext
» Eosinophilic vasculitis.
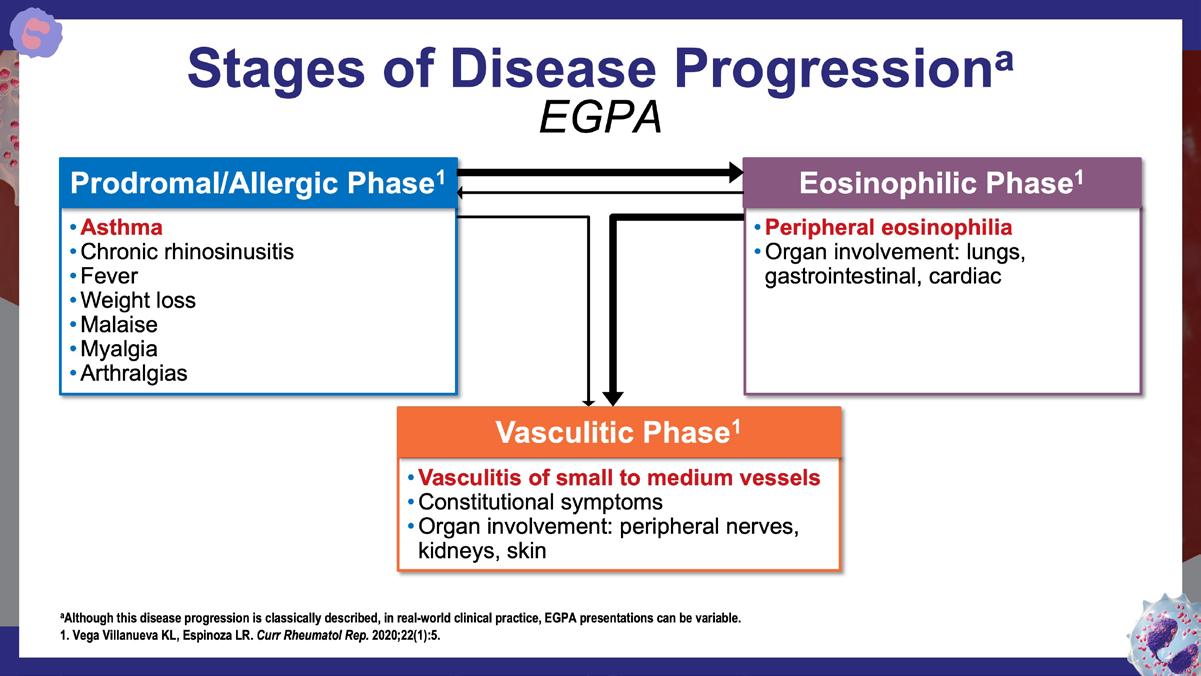
Vega Villanueva KL, Espinoza LR. Curr Rheumatol Rep. 2020;22(1):5.
https://link.springer.com/article/10.1007/s11926-020-0881-2
» Eosinophilic granulomatosis with polyangiitis: latest findings and updated treatment recommendations.
Watanabe R, Hashimoto M. J Clin Med. 2023;12(18):5996.
https://www.mdpi.com/2077-0383/12/18/5996
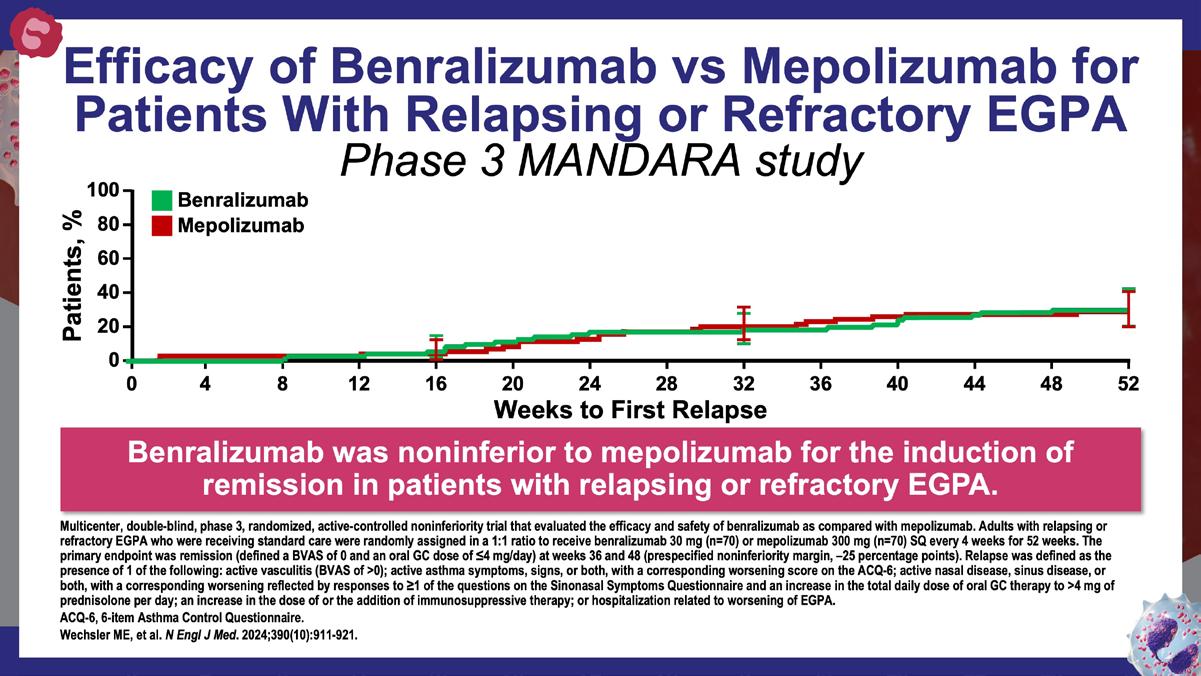
» Benralizumab versus mepolizumab for eosinophilic granulomatosis with polyangiitis.
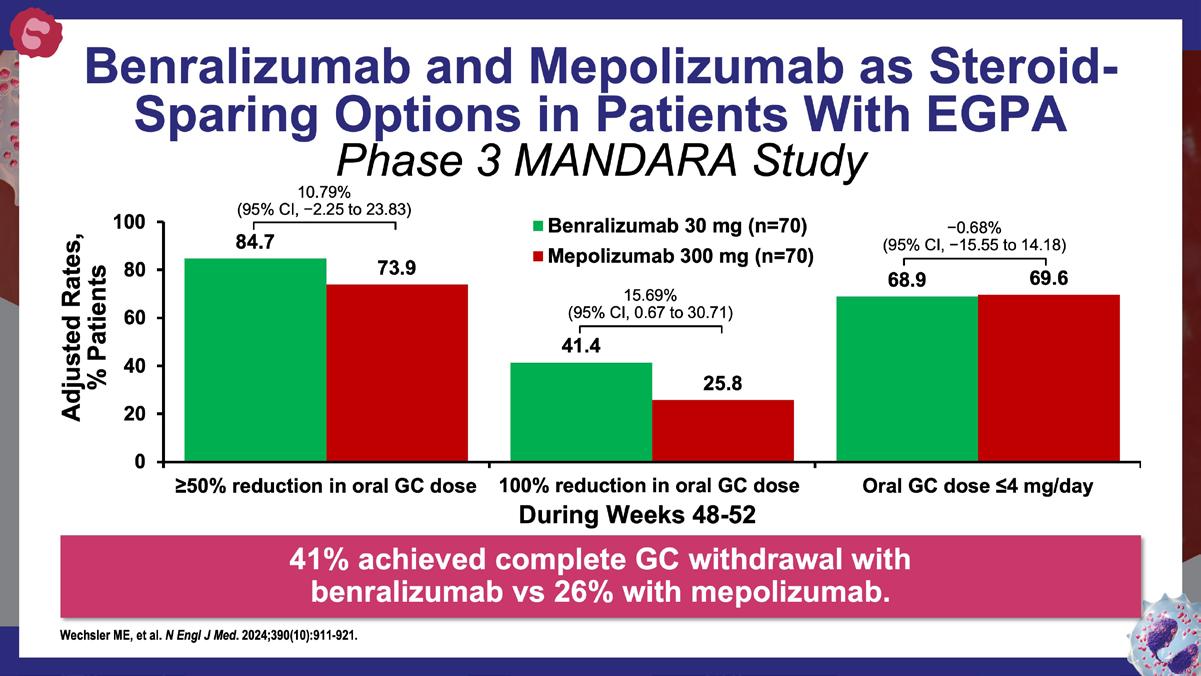
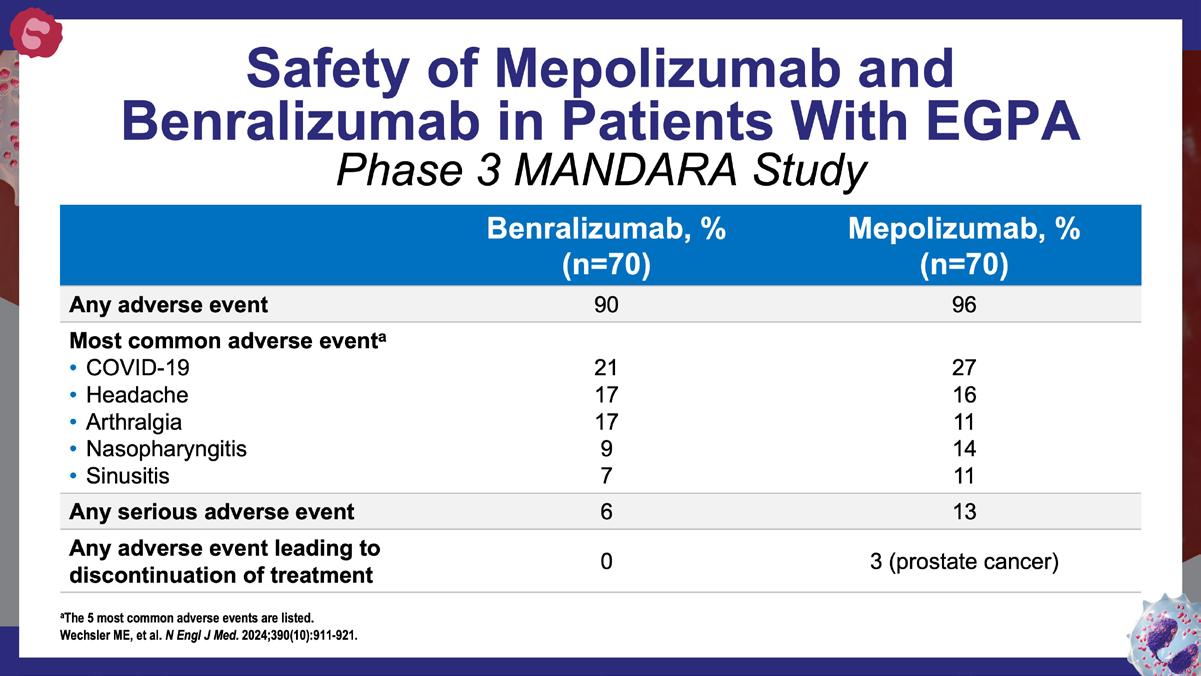
Wechsler ME, et al. N Engl J Med. 2024;390(10):911-921.
https://www.nejm.org/doi/10.1056/NEJMoa2311155?url_ver=Z39.882003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%20%200pubmed
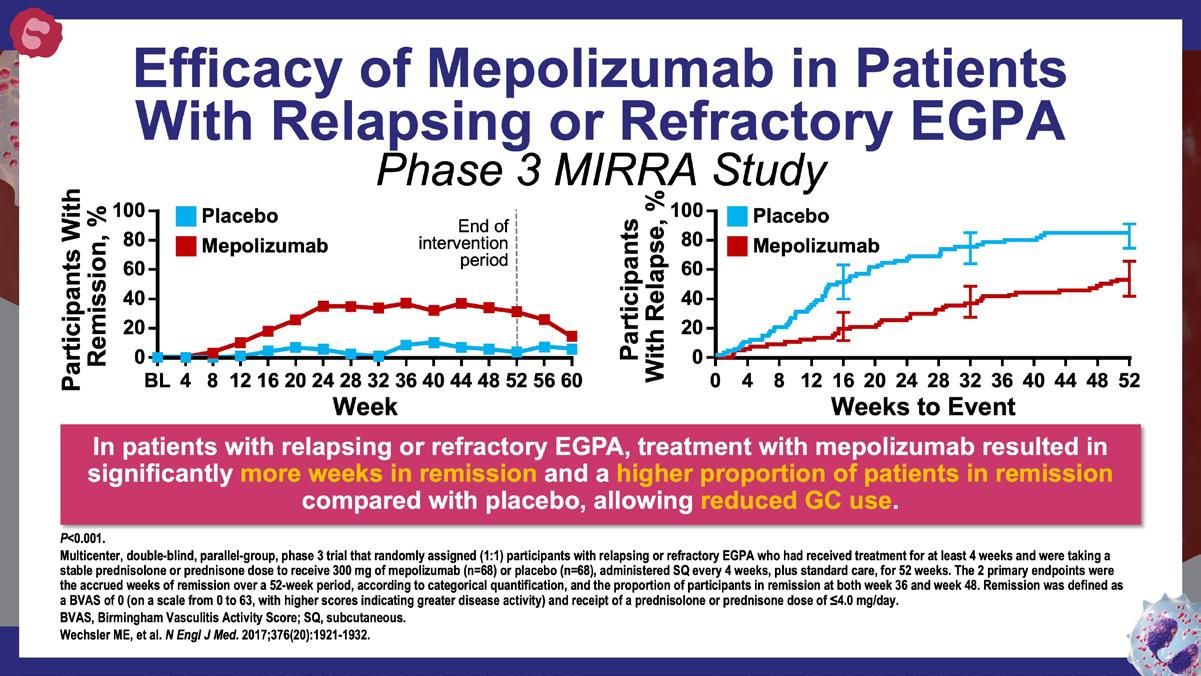
» Mepolizumab or placebo for eosinophilic granulomatosis with polyangiitis.
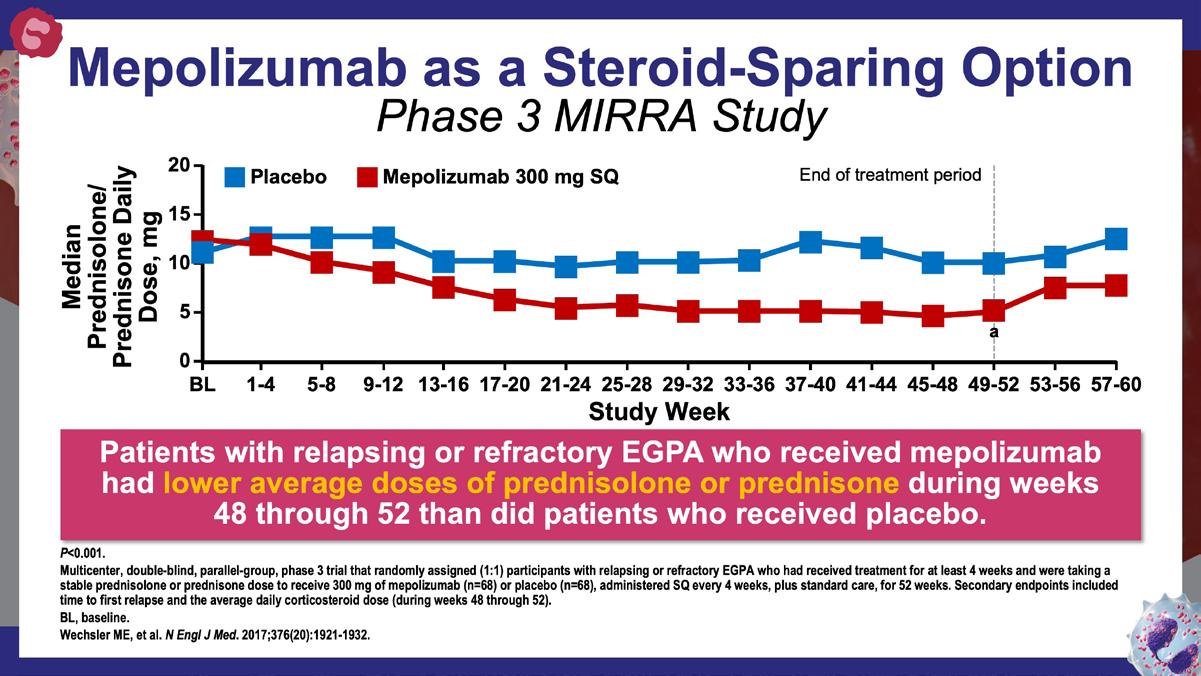
Wechsler ME, et al. N Engl J Med. 2017;376(20):1921-1932. https://www.nejm.org/doi/10.1056/NEJMoa1702079?url_ver=Z39.882003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%20%200www.ncbi. nlm.nih.gov
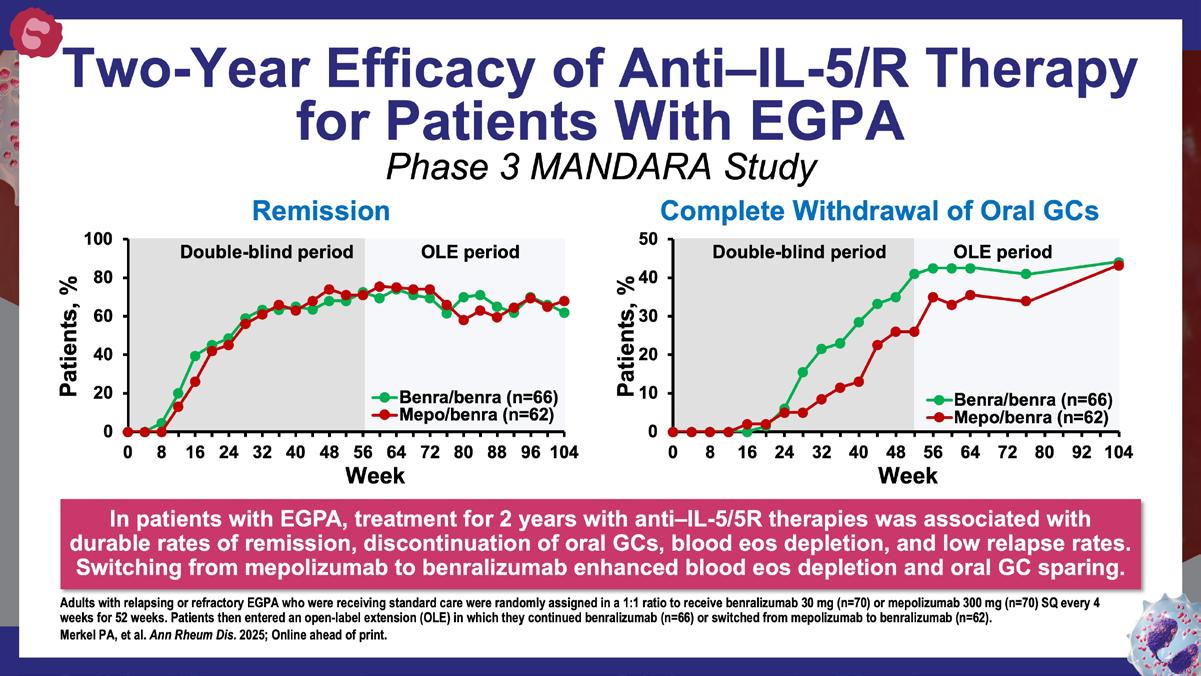
» Two-year efficacy and safety of benralizumab in the treatment of eosinophilic granulomatosis with polyangiitis.
Wechsler ME, et al. Am J Respir Crit Care Med. 2025;211:A5029. https://www.atsjournals.org/doi/abs/10.1164/ajrccm.2025.211. Abstracts.A5029
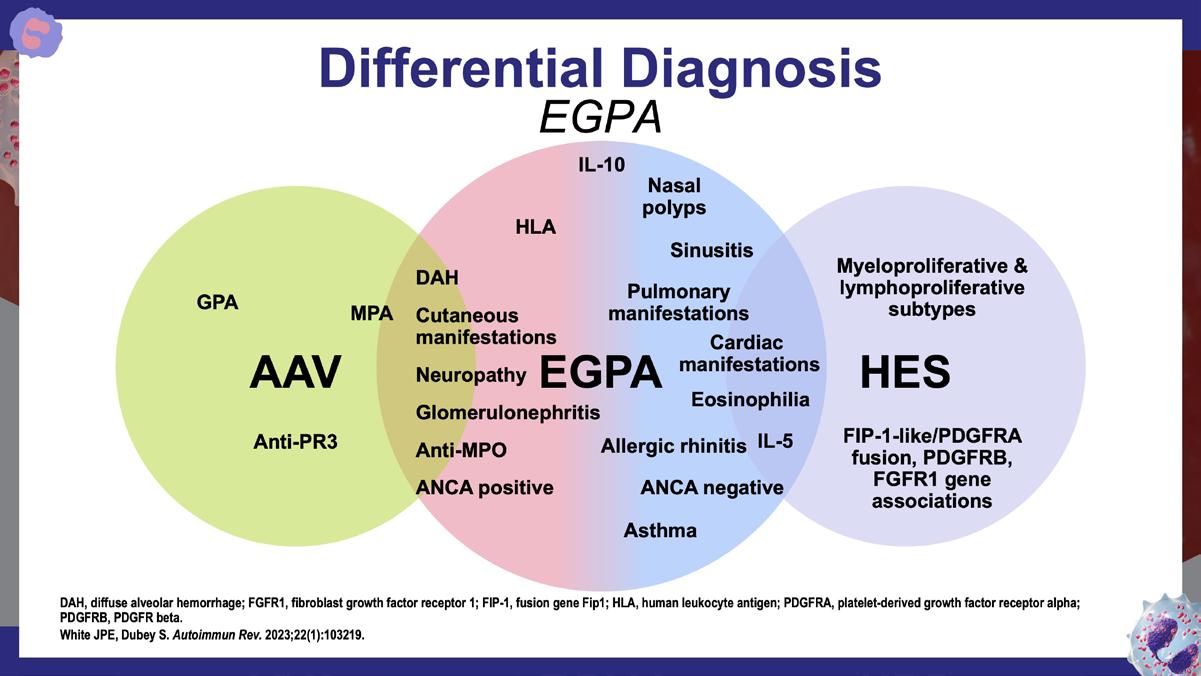
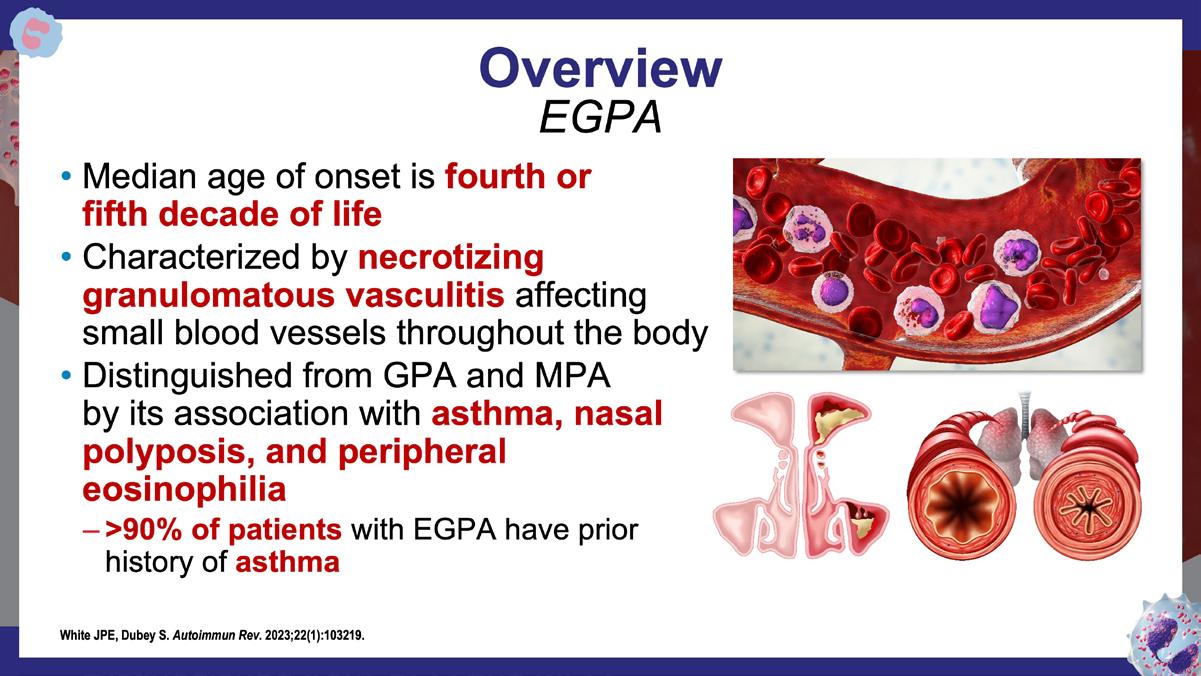
» Eosinophilic granulomatosis with polyangiitis: a review.
White JPE, Dubey S. Autoimmun Rev. 2023;22(1):103219. https://www.sciencedirect.com/science/article/pii/ S1568997222001896